Surgeon’s hands and career ‘saved’ by gene silencing drug
A leading hand surgeon with a rare inherited disease has been given new hope by the world’s first RNA interference drug to be approved for patients.

A pioneering gene-silencing drug may have saved the career of a top British surgeon with a rare hereditary disease that threatened to rob him of the use of his hands.
Carlos Heras-Palou expected his job as an orthopaedic specialist to end in just six months as the illness progressed and destroyed the nerves in his hands, rendering them useless.
But events took a dramatic turn after he joined an international trial and underwent 18 months of treatment with the drug, patisiran.
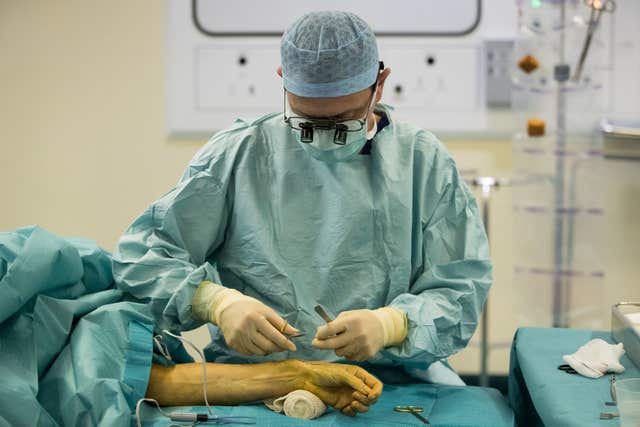
Freed from the destructive effects of the illness, Mr Heras-Palou’s peripheral nerves have started to recover and heal.
Patisiran is the first treatment of its type in the world to be approved for use in patients.
After getting the green light from the Food and Drugs Administration (FDA) in the US, it has now been licensed by European and UK regulators.
The cutting-edge drug employs a principle known as RNA interference to block activity of a rogue gene.
The disease, which can be inherited from either parent, causes sticky amyloid protein to build up in organs and around nerves, affecting vital functions such as limb movement, swallowing, vision and heartbeat. It also triggers chronic burning neuropathic pain.
Only about 100 people in the UK are thought to suffer from the disorder, which in some patients can shorten life expectancy to no more than five to seven years.
In a cruelly ironic twist, Mr Heras-Palou, 53, works as a hand surgeon in a specialist unit at the Royal Derby Hospital, knitting together delicate bones, tendons and nerves damaged by injury or disease.

He said: “My whole career depends on my hands and having a good hand function. Luckily I was diagnosed early and had mild symptoms, pins and needles and intermittent numbness. I’ve also had a lot of pain, problems eating and low blood pressure.
“Without this treatment the disease would have progressed and that would have been the end of my career.
“The disease often causes cardiomyopathy, affecting the heart muscle, and proves fatal.
“I told my colleagues what was happening and they were very supportive and were keeping an eye on me.
“I’m very well known in my field and have been practising a long time so my patients were perfectly safe. But in another six months my career would have been finished.”
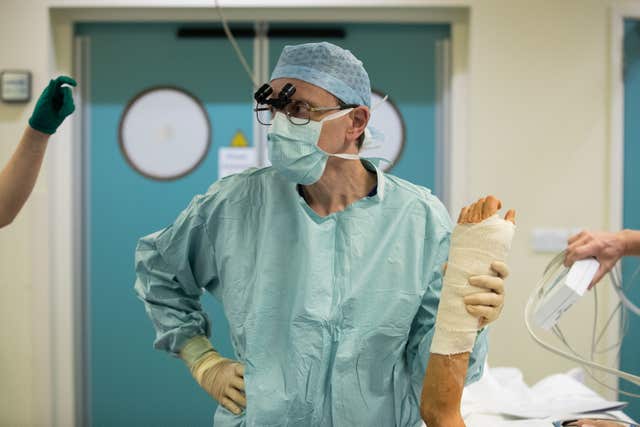
During the trial he had patisiran infused into his bloodstream every three weeks at the National Amyloidosis Centre, based at London’s Royal Free Hospital.
After a few months of therapy, the surgeon noticed that he felt better. Gradually the almost constant pain disappeared, and the prickly sensations in his hands began to fade. He could once again walk without difficulty.
“The treatment has saved my career, and my life,” said Mr Heras-Palou.
“I’m left with some numbness in my feet and weakness, and I still can’t run, but I can walk. I’m functioning very well.
“I haven’t got the pain and can eat normally so I’m not losing weight. I can sleep at night. I’ve been given hope and I’m seeing life in a different way.
“It is a ground-breaking advance, and it’s so selective there are practically no side-effects. It’s like the proverbial silver bullet.”

A report on the research, published in the New England Journal of Medicine, said disease progression was “halted or reversed” in patients who received the active drug.
As a trial participant, Mr Heras-Palou has been allowed to continue the very costly treatment, currently priced at around 400,000 US dollars (£308,618) per patient per year.
His sister, Isabel, who also suffers from the disease, was put on the drug as part of an earlier trial and is still improving.
Their mother, Catalina, another victim of hATTR amyloidosis, died from complications related to the disease four years ago.

“I think this is a really important drug.”
Patisiran, made by the US company Alnylam, is now under review by the National Institute for Health and Care Excellence (Nice), the body that approves or rejects new NHS treatments in England on grounds of cost-effectiveness. Final Nice guidance on the drug is expected next year.





