European regulators recommend Moderna Covid-19 jab
It is the second Covid-19 vaccine recommended by the European Medicines Agency.

European health officials have recommended the use of the Moderna Covid-19 vaccine.
The European Medicines Agency (EMA) has recommended granting a conditional marketing authorisation for the jab for adults.
British regulators – the Medicine and Healthcare products Regulatory Agency – are still conducting a review on the vaccine.
“It is a testament to the efforts and commitment of all involved that we have this second positive vaccine recommendation just short of a year since the pandemic was declared by WHO (World Health Organisation).
“As for all medicines, we will closely monitor data on the safety and effectiveness of the vaccine to ensure ongoing protection of the EU public.
“Our work will always be guided by the scientific evidence and our commitment to safeguard the health of EU citizens.”
The decision comes just days after the end of the Brexit transition period.
As part of the transition period, until the end of December 2020, Covid-19 vaccine candidates authorised via the EMA would automatically be valid in the UK.
It is the second jab recommended by the EMA after the Pfizer/BioNTech vaccine.
The Moderna vaccine was hailed as “tremendously exciting” when the US pharmaceutical company posted its phase three clinical trial results in November.
Its trial involved more than 30,000 people, half of whom received the vaccine and the other half received a placebo.
The vaccine demonstrated a 94.1% efficacy in the trial.
The trial also showed 90.9% efficacy in participants at risk of severe Covid-19.
Regulators in the US approved the jab and it is already being rolled out in the States.
The MHRA in the UK is conducting its own review into the vaccine.
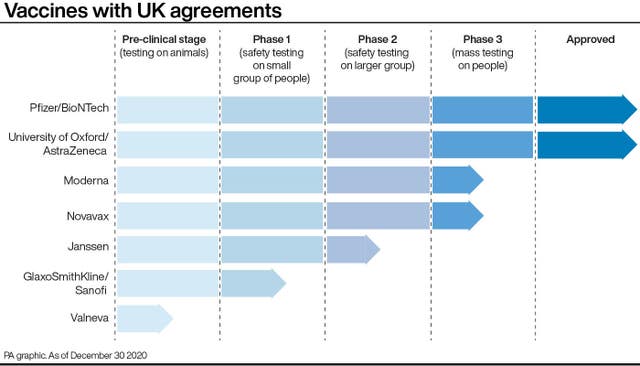
But the Government has said that the jabs won’t be available in Britain until “spring at the earliest” – should it be approved for use.
Commenting on the news Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said the more vaccines authorised “the better”.
He added: “We need many vaccines to be shown to be efficacious, to have no serious harms and to be of high quality.
“This is firstly because we need as many doses as we can get in this pandemic.
“Secondly, it is possible that we will find differences in some aspect of efficacy or safety between vaccines and having multiple options will enable this coronavirus to be suppressed worldwide.”





