AstraZeneca jab suspension did not deter UK public from getting it, study shows
Some EU nations banned use of the jab before the European Medicines Agency declared it is ‘safe and effective’.

The suspension of the AstraZeneca vaccine by some countries over blood clotting concerns had no impact on the UK public’s intention of getting the jab, new research suggests.
Several European countries including France, Germany and Italy suspended use of the vaccine last week, although they later said they would resume its rollout after the European Medicines Agency regulator concluded it is “safe and effective”.
University of Stirling researchers were collecting data for a wider project on fear and concerns relating to Covid-19 and examined whether negative news reports about the AstraZeneca jab resulted in “vaccine hesitancy”.
Comparing data from March 12-15, before the story reached its peak, with that from March 17, the day after the story peaked, they found no drop in intentions or attitudes towards getting the jab.
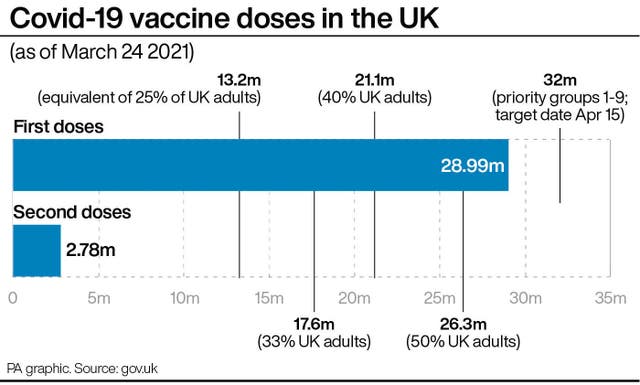
On March 17, the figure was 86.1%.
Dr David Comerford, of the university’s Behavioural Science Centre, said: “Our data provided time-lapse footage of the public’s response to the story.
“We found no effect of the press stories on intentions to take the vaccine or on intentions to refuse it. Furthermore, there was no change to the perceived costs and benefits of being vaccinated.
“This is not to say that the UK public were not concerned by the news. Google Trends data shows increasing search activity for the terms ‘vaccine’ and ‘safe’ as the AstraZeneca suspension story was unfolding, but that concern did not translate into mistrust of the vaccination programme in the UK.
“In fact, a record number of people took the vaccine on the following Saturday, March 20.”
Researchers looked at Google searches including the words “vaccine” and “safe” between February 21 and March 21 and found there was a “clear uptick in search activity coincident with the events and media reporting”, with search activity peaking on Monday March 15 and remaining high on March 16.
The report stated: “These data demonstrate that the blood clot/AZ suspension story entered the public consciousness and caused sufficient concern to prompt the UK public to seek information online.”
Data was collected from 546 people, with 241 responding between March 12-15 and 305 on March 17.
Researchers said the results suggest public confidence in the vaccination programme remains strong within the UK, but there is emerging evidence that confidence among European residents was damaged by these events.
The European Medicines Agency concluded there is no overall increase in the risk of blood clots with the vaccine, and in fact it is likely to reduce the overall risk of clots.
The regulator said its benefits in preventing Covid-19 hospital admission and death greatly outweigh potential risks.
Dr Comerford said: “An important question for future research is why the UK and European public responded differently and whether there are any lessons that can be learned to manage future scare stories.”





